SetLance activity is currently based on:
1) identification, production, characterization and preclinical development of antimicrobial peptides;
2) construction of peptide-based medical devices for the selective removal of bacteria and bacterial toxins from the blood of sepsis patients;
3) identification and characterization of anti-inflammatory peptides able to neutralize the over expression of pro-inflammatory cytokines;
4) characterization of peptides for oncologic applications.
The antimicrobial peptide SET-M33
The antimicrobial peptide SET-M33 is an optimized version of an artificial peptide sequence isolated from a random library and selected in the past years for its efficacy and stability. The peptide is synthesized in a tetra-branched form that gives high stability to circulating proteases. SET-M33 showed considerable activity against various multi-resistant Gram-negative bacteria, including clinical isolates of Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumanii and other Enterobacteriaceae. Moreover, when SET-M33 peptide is synthesized as D-stereoisomer it gains strong activity against the Gram-positive bacterium Staphylococcus aureus. SET-M33 was tested for its efficacy in models of sepsis, pneumonia and skin infections, showing a very promising activity profile.
References
Van der Weide H, Vermeulen-de Jongh DMC, van der Meijden A, Boers SA, Kreft D, Ten Kate MT, Falciani C, Pini A, Strandh M, Bakker-Woudenberg IAJM, Hays JP, Goessens WHF. Antimicrobial activity of two novel antimicrobial peptides AA139 and SET-M33 against clinically and genotypically diverse Klebsiella pneumoniae isolates with differing antibiotic resistance profiles. Int J Antimicrob Agents. 2019 Aug;54(2):159-166.
Castiglia F, Zevolini F, Riolo G, Brunetti J, De Lazzari A, Moretto A, Manetto G, Fragai M, Algotsson J, Evenäs J, Bracci L, Pini A, Falciani C. NMR Study of the Secondary Structure and Biopharmaceutical Formulation of an Active Branched Antimicrobial Peptide. Molecules. 2019 Nov 25;24(23):4290.
Falciani C, Zevolini F, Brunetti J, Riolo G, Gracia R, Marradi M, Loinaz I, Ziemann C, Cossío U, Llop J, Bracci L, Pini A. Antimicrobial Peptide-Loaded Nanoparticles as Inhalation Therapy for Pseudomonas aeruginosa Infections. Int J Nanomedicine. 2020 Feb 17;15:1117-1128.
Quercini L, Brunetti J, Riolo G, Bindi S, Scali S, Lampronti I, D’Aversa, E, Wronski S, Pollini S, Gentile M, Lupetti P, Rossolini GM, Falciani C, Bracci L, Pini A. An antimicrobial molecule mitigates signs of sepsis in vivo and eradicates infections from lung tissue. FASEB J. 2020 Jan;34(1):192-207.
Ritter D, Knebel J, Niehof M, Loinaz I, Marradi M, Gracia R, Te Welscher Y, van Nostrum CF, Falciani C, Pini A, Strandh M, Hansen T. In Vitro Inhalation Cytotoxicity Testing of Therapeutic Nanosystems for Pulmonary Infection. Toxicol In Vitro. 2020 Mar;63:104714.
Brunetti J, Carnicelli V, Ponzi A, Di Giulio A, Lizzi AR, Cristiano L, Cresti L, Cappello G, Pollini S, Mosconi L, Rossolini GM, Bracci L, Falciani C, Pini A. Antibacterial and Anti-Inflammatory Activity of an Antimicrobial Peptide Synthesized with D Amino Acids. Antibiotics (Basel). 2020 Nov 24;9(12):840.
Pollini S, Brunetti J, Sennati S, Rossolini GM, Bracci L, Pini A, Falciani C. Synergistic activity profile of an antimicrobial peptide against multidrug-resistant and extensively drug-resistant strains of Gram-negative bacterial pathogens. J Pept Sci. 2017 Apr;23(4):329-333
van der Weide H, Brunetti J, Pini A, Bracci L, Ambrosini C, Lupetti P, Paccagnini E, Gentile M, Bernini A, Niccolai N, Jongh DV, Bakker-Woudenberg IAJM, Goessens WHF, Hays JP, Falciani C. Investigations into the killing activity of an antimicrobial peptide active against extensively antibiotic-resistant K. pneumoniae and P. aeruginosa. Biochim Biophys Acta. 2017 Oct;1859(10):1796-1804.
Brunetti J, Falciani C, Bracci L, Pini A. Models of In-Vivo Bacterial Infections for the Development of Antimicrobial Peptide-based Drugs.Curr Top Med Chem. 2017; 17(5):613-619.
Brunetti J, Roscia G, Lampronti I, Gambari R, Quercini L, Falciani C, Bracci L, Pini A. Immunomodulatory and Anti-inflammatory Activity in Vitro and in Vivo of a Novel Antimicrobial Candidate. J Biol Chem. 2016 Dec 2; 291(49):25742-25748.
Brunetti J, Falciani C, Roscia G, Pollini S, Bindi S, Scali S, Arrieta UC, Gómez-Vallejo V, Quercini L, Ibba E, Prato M, Rossolini GM, Llop J, Bracci L, Pini A. In vitro and in vivo efficacy, toxicity, bio-distribution and resistance selection of a novel antibacterial drug candidate. Sci Rep. 2016 May 12;6:26077.
Falciani C, Lozzi L, Scali S, Brunetti J, Bracci L, Pini A. Site-specific pegylation of an antimicrobial peptide increases resistance to Pseudomonas aeruginosa elastase. Amino Aids. 2014, 46:1403-7.
Falciani C, Lozzi L, Pollini S, Luca V, Carnicelli V, Brunetti J, Lelli B, Bindi S, Scali S, Di Giulio A, Rossolini GM, Mangoni ML, Bracci L, Pini A. Isomerization of an antimicrobial peptide broadens antimicrobial spectrum to gram-positive bacterial pathogens. PlosOne. 2012, 7(10):e46259.
Pini A, Lozzi L, Bernini A, Brunetti J, Falciani C, Scali S, Bindi S, Di Maggio T, Rossolini GM, Niccolai N, Bracci L. Efficacy and toxicity of the antimicrobial peptide M33 produced with different counter-ions. Amino Acids. 2012, 43:467-73.
Pini A, Falciani C, Mantengoli E, Bindi S, Brunetti J, Iozzi S, Rossolini GM, Bracci L. A novel tetrabranched antimicrobial peptide that neutralizes bacterial lipopolysaccharide and prevents septic shock in vivo. Faseb J. 2010, 24:1015-22.
Pini A, Giuliani A, Falciani C, Fabbrini M, Pileri S, Lelli B, Bracci L. Characterization of the branched antimicrobial peptide M6 by analyzing its mechanism of action and in vivo toxicity. J Pept Sci. 2007,13:393-9.
Pini A, Giuliani A, Falciani C, Runci Y, Ricci C, Lelli B, Malossi M, Neri P, Rossolini GM, Bracci L. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob Agents Chemother. 2005, 49:2665-72.
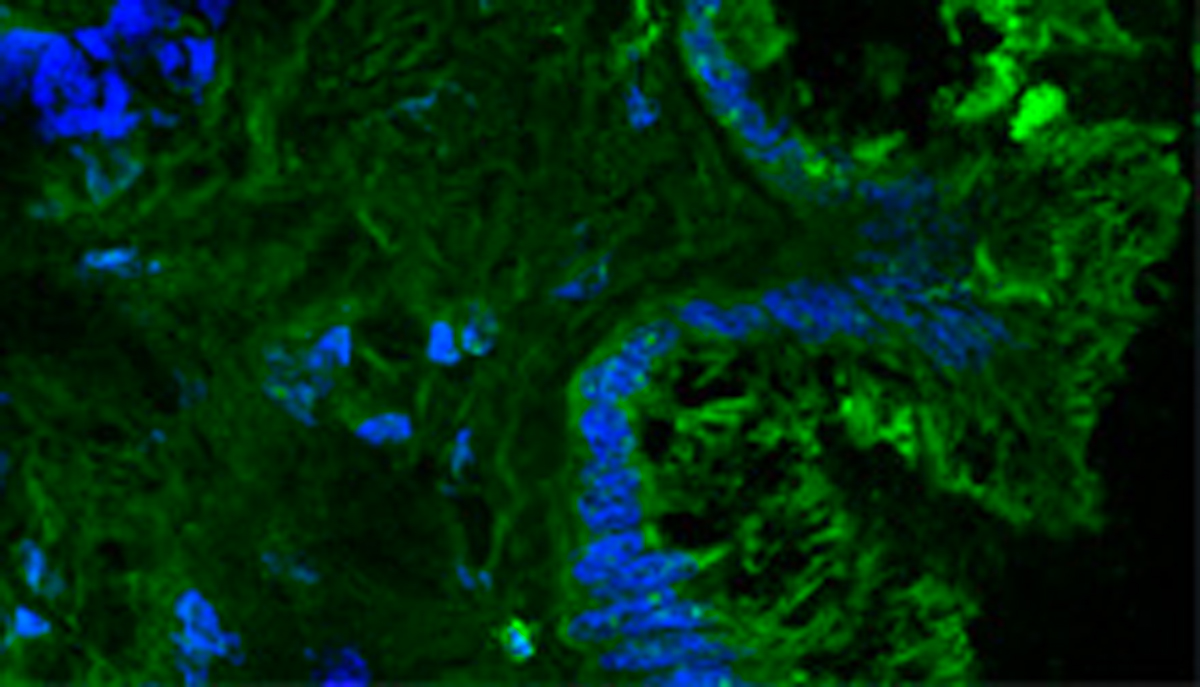
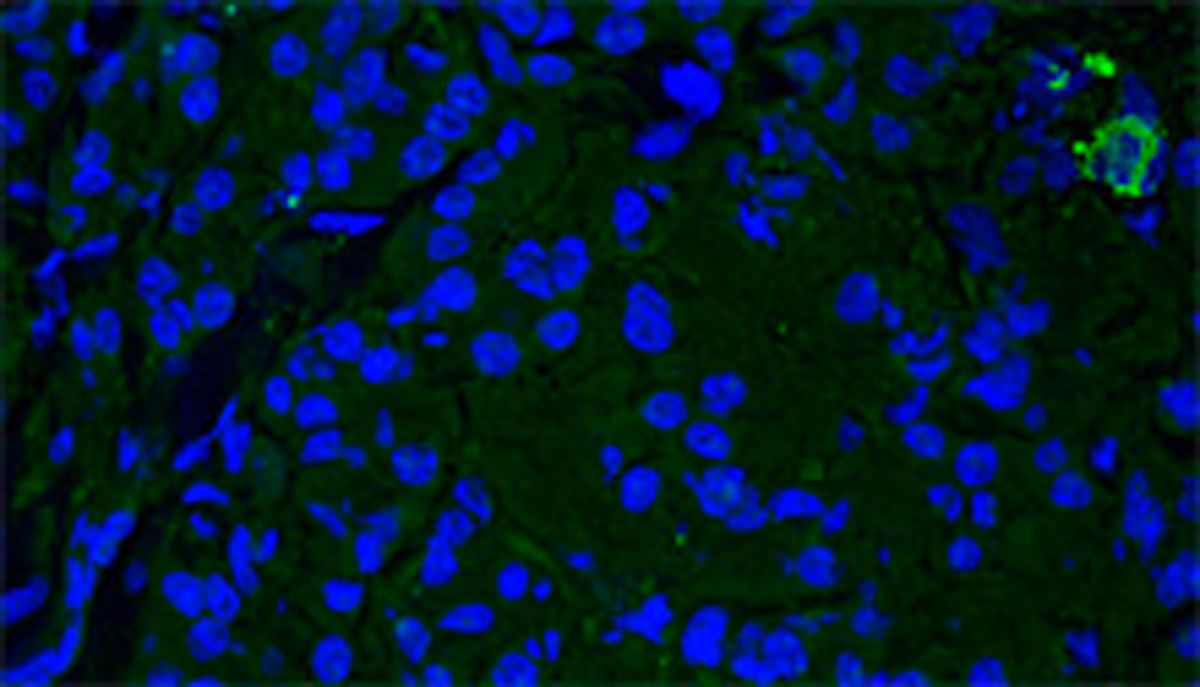
Branched neurotensin peptide as cancer theranostics.
Branched peptides bearing the sequence of human neurotensin (NT) are extremely selective for binding to different human cancer cells and tissues. A SetLance-patented tetrabranched peptide containing NT sequence (NT4) is conjugated to different functional units for selective imaging and killing of cancer cells. Unlike monomeric NT peptide NT4 efficiently discriminates between tumor and healthy tissue in human surgical samples of colon, pancreas adenocarcinoma and bladder cancer. Using NT4 conjugated to methotrexate or 5FdU, a significant reduction of tumor growth in mice was obtained.
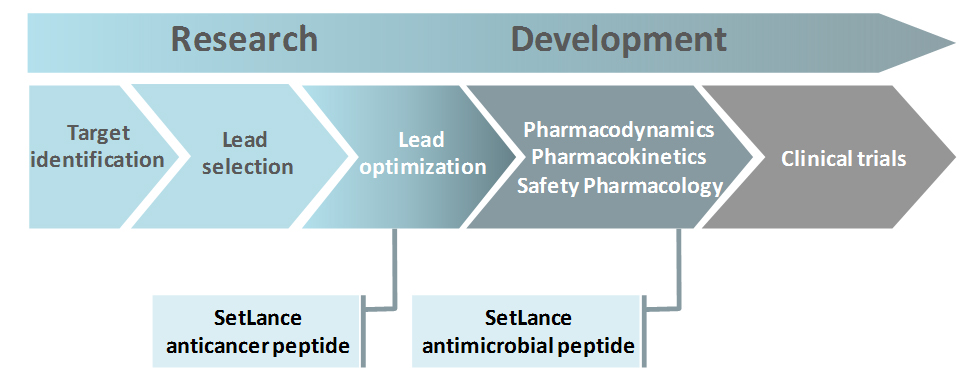
This peptide is currently in the lead optimization phase and expected to enter preclinical-pharmacological stage in the next future.
References
Depau L, Brunetti J, Falciani C, Scali S, Riolo G, Mandarini E, Pini A, Bracci L. Coupling to a cancer-selective heparan-sulfate-targeted branched peptide can by-pass breast cancer cell resistance to methotrexate. Oncotarget 2017.
Brunetti J, Depau L, Falciani C, Gentile M, Mandarini E, Riolo G, Lupetti P, Pini A, Bracci L. Insights into the role of sulfated glycans in cancer cell adhesion and migration through use of branched peptide probe. Sci Rep. 2016 Jun 3;6:27174.
Brunetti J, Pillozzi S, Falciani C, Depau L, Tenori E, Scali S, Lozzi L, Pini A, Arcangeli A, Menichetti S, Bracci L. Tumor-selective peptide-carrier delivery of Paclitaxel increases in vivo activity of the drug. Sci Rep. 2015 Dec 2; 5:17736.
Brunetti J, Falciani C, Lelli B, Minervini A, Ravenni N, Depau L, Siena G, Tenori E, Menichetti S, Pini A, Carini M, Bracci L. Neurotensin branched peptide as tumor-targeting agent for human bladder cancer. Biomed Res Int. 2015; 2015:173507.
Falciani C, Brunetti J, Lelli B, Ravenni N, Lozzi L, Depau L, Scali S, Bernini A, Pini A, Bracci L. Cancer selectivity of tetrabranched neurotensin peptides is generated by simultaneous binding to sulfated glycosaminoglycans and protein receptors. J Med Chem. 2013, 56:5009-18.
Falciani, C.; Accardo, A.; Brunetti, J.; Tesauro, D.; Lelli, B.; Pini, A.; Bracci, L.; Morelli, G. Target-selective drug delivery through liposomes labeled with oligobranched neurotensin peptides. Chem-MedChem 2011, 6, 678−685.
Falciani, C.; Brunetti, J.; Pagliuca, C.; Menichetti, S.; Vitellozzi, L.; Lelli, B.; Pini, A.; Bracci, L. Design and in vitro evaluation of branched peptide conjugates: turning nonspecific cytotoxic drugs into tumor-selective agents. ChemMedChem 2010, 5, 567−574.
Falciani, C.; Lelli, B.; Brunetti, J.; Pileri, S.; Cappelli, A.; Pini, A.; Pagliuca, C.; Ravenni, N.; Bencini, L.; Menichetti, S.; Moretti, R.; De Prizio, M.; Scatizzi, M.; Bracci, L. Modular branched neurotensin peptides for tumor target tracing and receptor-mediated therapy: a proof-of-concept. Curr. Cancer Drug Targets 2010, 10, 695−704.
Falciani, C.; Fabbrini, M.; Pini, A.; Lozzi, L.; Lelli, B.; Pileri, S.; Brunetti, J.; Bindi, S.; Scali, S.; Bracci, L. Synthesis and biological activity of stable branched neurotensin peptides for tumor targeting. Mol. Cancer Ther. 2007, 6, 2441−2448.